Our laboratory focuses on the understanding of the ecology, immunology and pathology of wildlife infectious diseases. Most of the pathogen (parasite) diversity in the planet is associated to wildlife species. Therefore, from an ecological and global health perspective, it is critical to know and understand the drivers of this pathogen diversity. While most of our approaches and research questions are based on the microbial and host side of the infectious disease triad, we also collaborate with diverse groups that focus on the impact of environmental change on the health of animals and their susceptibility to infectious diseases.
The Immune system as key player in the dynamics of wildlife infectious diseases
The immune response is one of the most important host traits impacting the dynamics of infectious diseases in nature. We believe that wildlife study systems provide unique models to understand different immunological processes in the context in which they evolved. That is why our main line of research focuses on the ontogeny of the immune system and how consistent inter-individual differences in the immune response (or “immunotypes”) are generated within a population. In this context, we seek to identify the main factors that impact immune responses in nature from early-life programming to senescence, and to understand the pathophysiological mechanisms behind the processes.
Currently we work with one primary study system (fur seals at Chilean Patagonia) but also collaborate in other wildlife disease systems in North America and South Africa
Study systems
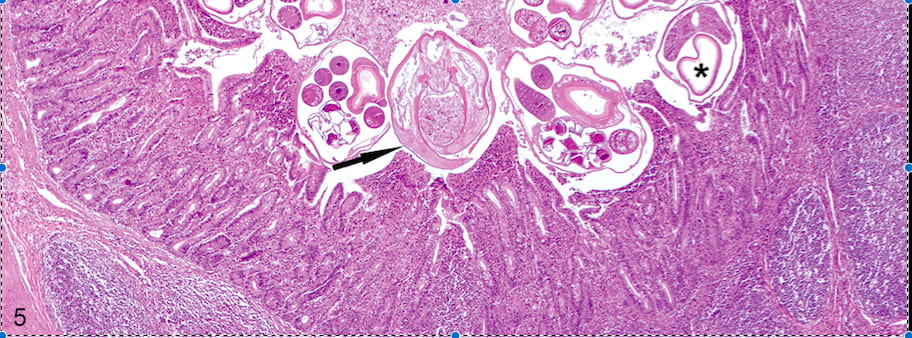
Diseases of fur seals at Chilean Patagonia. This is a long-term research program carried out at Guafo Island, Northern Chilean Patagonia. At this location we have been studying the infectious diseases of South American fur seals for 10 years, focusing on the host and environmental drivers of the infection and its consequences at the population level.
Tuberculosis and helminths in African buffalo. Our laboratory collaborates in this long-term project, lead by Dr Vanessa Ezenwa that seeks to understand the impact of helminth co-infection on the dynamics and evolution of TB. We have used this system to explore some key immunological questions such the impact of immune variability on infection susceptibility and the drivers and consequences of immunosenescence.